This is an AI for Health MSc project. Students are eligible to receive a monthly reimbursement of €500,- for a period of six months. For more information please read the requirements.
Clinical problem
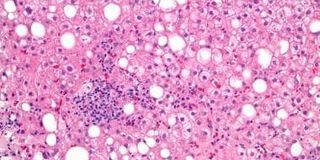
Non-alcoholic fatty liver disease (NAFLD) is an enormous global burden with an estimated prevalence of 20-30% worldwide. NAFLD, defined as excessive hepatic lipid accumulation in absence of alcohol use, can progress into non-alcoholic steatohepatitis (NASH), a disease characterized by hepatic inflammation and fibrosis (‘scarring’). Although only recently acknowledged, NASH can have severe consequences, such as a twofold increased risk of cardiovascular diseases, the development of liver cirrhosis with liver failure, and liver cancer. To date, the gold standard to diagnose and stage severity of NAFLD-NASH is a liver biopsy. A liver pathologist evaluates the histopathological slide and determines the severity of the disease by assessment of the amount of fat (or: ‘steatosis’), inflammation, hepatocyte ballooning and fibrosis. Unfortunately, there are still major issues in the histopathological assessment of NAFLD-NASH. The first major problem is an enormous interobserver variability. The severity that two independent pathologists ascribe to a histopathological slide differs in more than 50% of cases. As one can imagine, this severely influences a patient’s health, treatment and prognosis. In addition, the lack of consistency also seriously influences the research in NAFLD-NASH, where improvements due to novel therapies can be masked by interobserver variability. Second, a major problem and practical inevitability in the assessment of NASH histopathology by humans, is the categorical nature of severity score systems. When a histopathological slide contains 32% steatosis it is appraised one point, but when it is estimated to be 34%, the biopsy gets appraised two severity points. Therefore, this categorical nature implies a certain inaccurateness in the determination of the severity of the disease, and thereby has major consequences for both patients and the research field.
Solution
Although, the NAFLD-NASH research field cries out for the implementation of artificial intelligence (AI) in histopathology, this is still in early stages. Yet through the development and validation of a deep learning model, we can develop a standardized histopathological assessment with a continuous scale. Furthermore, AI models encompass the future of histopathological examination, and the developed models in the NAFLD-NASH domain can probably be extrapolated to the histopathological assessment of other diseases.
Data
Currently, we have a growing cohort of patients with NAFLD-NASH, complete with medical records, imaging details, and scanned histopathological slides. In total, this database contains 60+ individuals with NAFLD-NASH. In addition, we have access to a cohort of ± 250 participants with liver biopsies, which can be used for further validation of the model.
Approach
Students will be supervised by a team of NAFLD-NASH experts from the Amsterdam UMC (dr. Onno Holleboom, and drs. Quinten Augustijn) and a team of experts in the field of deep learning in histopathology from the Radboud University (dr. ir. Geert Litjens and prof. dr. Jeroen van der Laak). Primarily based in Nijmegen, the student will develop a deep learning model for the assessment of NAFLD-NASH. The student will be trained by a liver pathologist in the assessment of NAFLD-NASH. Thereafter the student will build a supervised model upon the histopathological slides of 60-100 patients. When successful, the model will further be validated in larger cohorts. We expect that the thesis will results in a significant scientific publication.
Requirements
Students Artificial Intelligence, Data Science, Computer Science, Bioinformatics, Biomedical Engineering or similar in the final stages of their Master education. You should be proficient in Python programming and have a theoretical understanding of deep learning architectures. Experience with medical images is beneficial.
Information
- Project duration: 6 - 9 months
- Location: Radboud University Medical Center
- For more information or to apply for this project, please contact Geert.Litjens@radboudumc.nl.
